- Knowledge
Patent Invalidation System in Brazil: Legal Framework and Challenges in Emerging Markets
As the largest economy in Latin America, Brazil's patent system integrates the traditional civil law system with local public health policies, forming a unique invalidation mechanism. According to the revised Industrial Property Law of 2023 (Lei n º 9279/1996), the patent invalidation procedure has dual track characteristics of administrative examination and judicial relief. This article provides a deep analysis of legal basis, procedural path, typical cases, and cross-border enterprise response strategies.

Legal reasons for patent invalidity (Article 50 of the Industrial Property Law)
A patent right may be declared invalid based on any of the following conditions:
Prior to the priority date, publicly disclose globally (including use, sale, and publication). Special rule: Public experiments conducted within Brazil are entitled to a 12-month grace period (Article 12), but must be registered with INPI (Patent Office) in advance.
Adopting technical problem-solving standards: If the existing technology combination constitutes a direct solution for technical personnel in this field, it is deemed invalid. Unlike Europe and America, Brazil accepts preprints of academic papers (such as arXiv. org) as existing technology (Samsung v. Ericsson, 2022).
It needs to be proven that it can be implemented on a large scale within Brazil. In the 2023 BASF pesticide patent case, it was ruled invalid due to the lack of explanation of tropical climate adaptation schemes (such as rainy season storage conditions).
Automatically invalid in the following areas:
Human cloning technology
Genetic modification of Amazon specific plants (requires permission from the Genetic Heritage Management Committee)
Genetically modified organisms that violate the Biosafety Law (Lei n º 11105/2005)

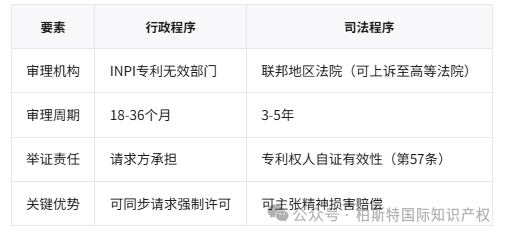
Dual track procedure: administrative review vs. judicial litigation
Core process:
1. Compulsory administrative prerequisites:Invalid requests must first be submitted to INPI, with an average review period of 24 months (with a backlog of 11200 cases in 2023)
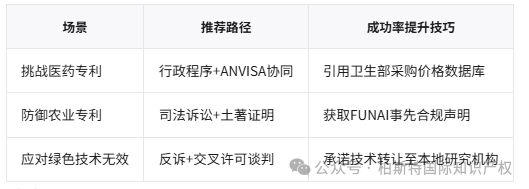
2. Special review of public health:Drug patents require an opinion from the National Health Supervision Administration (ANVISA) (Article 229-C)
3. Rules of Evidence:Only accept Portuguese documents or consular certified translations
Typical case:
The 2021 Gilead antiviral drug patent case was declared invalid by INPI due to ANVISA's determination that it posed a threat to the sustainability of Brazil's public health system.
Program features:
Data pivot:
2022 INPI invalidation success rate: 38%
Judicial overturning of administrative decisions: 52% (mainly due to procedural flaws)

Institutional uniqueness: balance between public health and technological sovereignty
According to Article 229-C, even if INPI has granted a patent, ANVISA may still reject it based on the following reasons:
Drug pricing exceeds national income by 30%
Local production commitment not fulfilled
Practical impact: 79% of imported anti-cancer drug patents will be challenged as a result in 2023.
For clean energy patents (such as biofuels), the invalidation process is shortened to 12 months, and the requesting party only needs to provide preliminary evidence (Article 70-A).
If the invention originates from indigenous knowledge (such as Amazon plant applications), an informed consent certificate from FUNAI (National Indian Foundation) must be submitted, otherwise it will be invalid (Natura v. Yanomami Tribe, 2020).

Enterprise Practice Guide: Three Steps of Risk Prevention and Control
1. Localized disclosure requirements
The implementation example needs to include Brazilian biological samples (such as tropical soil testing data)
Drug patents require submission of clinical data from public hospitals (ANVISA Resolution No. 9)
2. Key points of language compliance
Priority documents must be submitted with an officially certified Portuguese translation within 90 days
To avoid ambiguity in literal translation terms (such as' about 10 'in English should be translated as' aproximate 10' instead of '10% exam')
High reliability evidence:Brazilian scientific journals (such as SciELO) and INPI search reports
Restricted evidence:Foreign court judgments (requiring authentication by the Supreme Court), testimony from non Portuguese speaking experts
Killer evidence:Patent holder's failure to implement declaration (automatic invalidation risk after triggering compulsory license)

Latest news and cross-border enterprise warning
INPI has enabled an AI system to identify patent jungles with a misjudgment rate of up to 23% (due to algorithm neglect of technological synergies), resulting in batch invalidation of biopharmaceutical patents.
Translation trap:32% of invalid cases in 2023 have been reduced in scope due to approximately accurate translation
Program overdue:Failure to respond to INPI review comments within 30 days (legal time limit is only 1/3 in Europe and America)
Traditional knowledge omission:The traditional Chinese medicine formula does not declare the history of similar plant applications on Amazon
Brazil plans to implement a fast track mechanism for BRICS patents, but the invalidation process will still be subject to domestic standards, which may lead to a decrease in the stability of authorized patents.




