- Knowledge
Patent Invalidation System in Canada: Legal Framework and Practical Guidelines
The core of the patent system lies in balancing innovation incentives with public interests, and the stability of patent rights directly affects this balance. As a common law country, Canada has established a patent invalidation mechanism that combines efficiency and fairness. This article analyzes this system from multiple dimensions, including legal basis, procedural path, typical cases, and practical strategies.

Legal Framework and Invalid Basis
The current Patent Act in Canada clearly stipulates that the validity of patent rights may be challenged in litigation proceedings before federal courts or the Patent Re examination Board of Canada. The core legal basis for invalid applications mainly revolves around the following elements:
According to Article 28.2 of the Patent Law, if an invention has been publicly used, sold, or fully disclosed through publications or other means before the application date (even if the disclosure comes from the patentee themselves), it constitutes loss of novelty. The federal court established a clear directional standard in the 2018 AstraZeneca v. Apotex case, requiring existing technology to directly and unambiguously disclose all patented technical features.
In the 2008 Sanofi Synthelabo v. Apotex case, the Supreme Court systematically outlined the four step test method: confirming the existing technological foundation, identifying invention differences, evaluating whether it is an obvious attempt, and considering supporting evidence such as commercial success. The court emphasized the need to avoid retrospective judgments and focus on whether the solution path to technical problems is predictable.
Canada adopts a commitment verification standard for practicality: the use commitment recorded in the patent specification must be supported by experimental data. If the patentee claims a use beyond the scope of the specification, it may trigger a utility defect under Article 2 (1), as in the 2017 Eli Lilly v. Mylan case where the patent was invalidated due to failure to verify specific therapeutic effects.

The dual track path of ineffective challenges: a comparison between judicial and administrative procedures
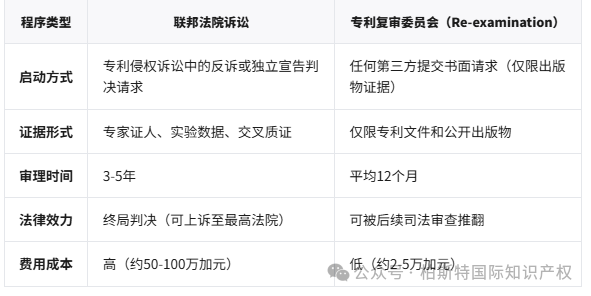
Startup method:Request for counterclaim or independent declaration of judgment in patent infringement litigation
Evidence Form:Expert witnesses, experimental data, cross examination
Trial time:3-5 years
Legal effect:Final judgment (appealable to the Supreme Court)
Cost of expenses:High (approximately 500000 to 1 million Canadian dollars)
Startup method:Any third party submitting a written request (limited to publication evidence only)
Evidence Form:Only applicable to patent documents and public publications
Trial time:Average of 12 months
Legal effect:Can be overturned by subsequent judicial review
Cost of expenses:Low (approximately 20000 to 50000 Canadian dollars)
Strategic suggestion:Generic drug companies often quickly clear patent barriers through re examination procedures, while complex technical disputes are more suitable for in-depth judicial verification. However, it should be noted that the 2021 ViiV Healthcare case shows that the court may overturn administrative rulings, so a combination of "review litigation" strategies is often used.

Institutional specificity: Canada
Key points of practice
Using a 'Balance of Probability' (>50% likelihood), which is lower than the 'Clear and Convincing Evidence' standard in the United States, the ineffective success rate is approximately 35% -40%.
In the 2020 Janssen v. Teva case, although the drug met the standards in commercial production, it was deemed invalid due to insufficient information disclosure as the impact of storage temperature on stability was not clearly stated in the instructions.
In recent years, there have been frequent disputes over vaccine and gene therapy patents, and courts are paying more attention to technical details. In the 2022 Pfizer vaccine case, the creativity of the lipid delivery system was deemed non obvious, highlighting the defensive value of technical complexity.

Practical Guidelines for Enterprise Response
Research and development records with clear timestamps
Contemporary technical literature from third-party journals
Competitors' public product analysis report
Prioritize hiring academic authorities (such as university professors) whose testimony is more persuasive in cross examination than that of corporate experts.
Canada has not joined the Patent Law Treaty, so special attention should be paid to:
Application for Translation Accuracy (Mechanical Translation May Trigger Article 53 'Misleading Statements' Invalid)
Priority linkage (one month less grace period than the United States)




