- Knowledge
A Comprehensive Analysis of the Patent Invalidation System in Mexico: Legal Framework, Administrative Procedures, and Practical Strategies
The invalidation system of patents in Mexico is based on the Industrial Property Law (Ley de la Propiedad Industrial, LPI), with the Mexican Industrial Property Institute (IMPI) leading the administrative process and judicial review as a supplement. As an important market in Latin America, Mexico's patent invalidation process is both efficient and adversarial, and has become a key battlefield for multinational corporations due to increasingly prominent technological disputes in the fields of medicine and energy. This article systematically analyzes the legal basis, procedural characteristics, and practical strategies of patent invalidity in Mexico.
PART .01
Legal Basis: Legal Reasons for Patent Invalidation (Articles 152 and 153 of the LPI)
According to Article 152 of the LPI, the circumstances in which a patent may be declared invalid include:
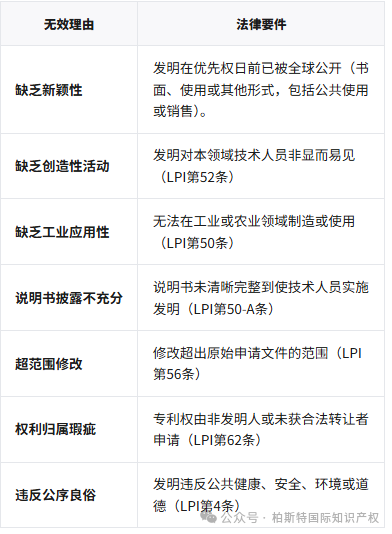
Special rules:
Pharmaceutical patents:Patents must comply with the efficacy standards of the World Health Organization (WHO), otherwise they may trigger public interest clauses.
Plant species:According to the Federal Plant Variety Act, new plant varieties are not patentable objects (Article 4 II of the LPI).
PART .02
Administrative invalidation procedure:
IMPI led monorail system
Invalid requests need to be submitted to the Mexican Industrial Property Institute (IMPI), and the core process is as follows:
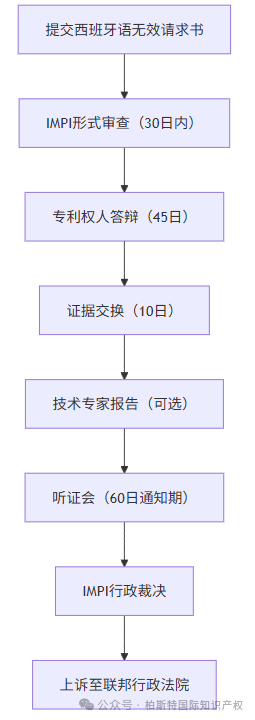
Program timeliness:The average processing time is 12-18 months, with complex cases extending to 24 months.
cost:The administrative procedure fee is approximately 3000-10000 US dollars (excluding legal fees).
IMPI may designate technical experts (Perito) to issue reports based on their authority or upon request, especially in complex fields such as medicine and chemical engineering.
Partial invalidity: IMPI may declare certain claims of the patent invalid.
Correction procedure: The patentee may request modification of the claims in the invalidation procedure (Article 171 of LPI), but the scope shall not be expanded.
PART .03
Judicial review: the final review power of federal courts
Appeal channel:Those who are dissatisfied with the IMPI ruling can appeal to the Federal Administrative Court (TFJFA) and ultimately to the Supreme Court (SCJN).
Scope of review:The court only reviews the legality of administrative procedures (such as evidence adoption and procedural compliance) and does not re-examine technical facts.
PART .04
The linkage between invalid procedures and infringement lawsuits
Invalid defense:Defendants can claim patent invalidity in infringement lawsuits, but Mexican courts usually suspend proceedings and wait for IMPI rulings (Article 379 of LPI).
Temporary ban risk:If the court issues a temporary injunction (Medidas Caudelares) in an infringement lawsuit, even if the patent is ultimately invalidated, the defendant must make a separate claim.
PART .05
Rules of Evidence and Practical Points
Burden of proof:The invalid requester shall bear the burden (based on the principle of "whoever asserts, bears the evidence").
Core Evidence Requirements:
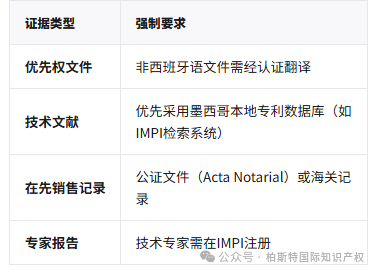
Localized evidence: Mexican courts have a high degree of acceptance of local technical literature, such as academic journals.
PART .06
Practical Strategies and Industry Guidelines
Pre objection: Using the pre authorization objection procedure (Oposici ó n Previa, LPI Article 120) to prevent patent authorization at a lower cost.
Invalid counterclaim: Submit an invalid request within 30 days after receiving the infringement complaint, striving for the advantage of procedural parallelism.
Notarization of therapeutic efficacy data:Provide data from WHO or the Mexican Ministry of Health (COFEPRIS) to demonstrate the lack of new therapeutic effects (such as generic drug equivalence reports).
International conventions utilize:Quoting the Doha Declaration to defend against public health crises and promoting the linkage between compulsory licensing and invalidity.
Local proxy enforcement: Invalid programs must be submitted by a registered patent agent (Agente) in Mexico.
Certified translation budget: Non Spanish evidence requires official translation, which accounts for 15% -30% of the cost.




